Contents
Bio-Medical Hazardous waste
Introduction:
Biomedical waste originates from human or animal health care, medical research, medical teaching facilities, funeral establishments, laboratories and other facilities. A portion of that waste stream is infectious or potentially infectious and presents a potential hazard to the public health and the environment.
Infectious or potentially infectious biomedical waste is a contaminant under the Environmental Protection Act (EPA) and must be managed as a hazardous waste.
The objective of these guidelines is:![]()
- To provide uniform standards for the segregation, management and disposal of infectious or potentially infectious biomedical waste.
- To reduce the incidence of health care worker and the public from contacting a disease or injury from biomedical waste.
- To provide guidance to the health care system on the opportunities for waste minimization and the reduction of air contamination from incineration of biomedical waste.
Medical/bio hazardous waste is defined as waste that requires inactivation of the biological material in an approved manner prior to final disposal, and includes but is not limited to the following discarded items:
- Human cells and tissues
- Organisms or cells with recombination DNA
- Cultures and stocks of infectious agents
- Potentially infectious agents (e.g., bacteria, viruses, fungi, prions)
- Biological material that may contain potentially infectious agents
- Toxins (e.g., snake venom)
- Live and attenuated vaccines
- Blood, blood products, and other potentially infectious materials that may contain human blood-borne pathogens
- Carcasses and tissues
- Soil, plants, and pathogens controlled by the agriculture department.
- Lab ware and other items that have come into contact with the aforementioned waste steams (e.g., contaminated plastic pipettes, pipette tips, petri dishes, centrifuge tubes, tubes, disposable gloves, and wipes)
Definitions:
Many terms are used to identify and characterize biomedical waste, such as: bio hazardous, pathological, and infectious. These terms are often used interchangeably without clearly defining their subtle differences and similarities. To assist the reader these terms are defined below. For the purpose of this document biomedical waste will be used as the general term. Where the waste may be defined more specifically and require special treatment, this procedure will specify the requirements.
Bio hazardous waste:
Waste that is known or suspected to contain infectious material or which because of its physical or biological nature may be harmful to humans, animals, plants or the environment
Infectious waste:
Waste which contains microorganisms in sufficient quantity which could result in the multiplication and growth of those microorganisms in a host.
Pathological waste:
Any waste which contains microorganisms capable of causing disease.
Agent
A pathogen that can cause human or animal disease including bacteria, mycoplasma, fungi, viruses and parasites.
Autoclave
A device that uses high-pressure, high temperature steam sterilization for the destruction of bacteria, spores and other infection-causing organisms.
a) Human Anatomical Waste
This consists of human tissues, organs, and body parts, but does not include teeth, hair, and nails.
b) Animal Waste
This consists of all animal tissues, organs, body parts, carcasses, bedding, fluid blood and blood products, items saturated or dripping with blood, body fluids contaminated with blood, and body fluids removed for diagnosis or removed during surgery, treatment or autopsy, unless a trained person has certified that the waste does not contain the viruses and agents listed in Risk Group 4 (see table 1). This excludes teeth, hair, nails, hooves, and feathers.
c) Microbiology Laboratory Waste
This consists of Laboratory cultures, stocks or specimens of microorganisms, live or attenuated vaccines, human or animal cell cultures used in research, and laboratory material that has come into contact with any of these.
d) Human Blood and Body Fluid waste
This consists of human fluid blood and blood products, items saturated or dripping with blood, body fluids contaminated with blood, and body fluids removed for diagnosis during surgery, treatment or autopsy. This does not include urine or feces.
e) Waste Sharps
Waste sharps are clinical and laboratory materials consisting of needles, syringes, blades, or laboratory glass capable of causing punctures or cuts.
f) Cytotoxic Waste
The term is commonly used to refer to pharmaceuticals used in treating cancer, e.g., antineoplastics or chemotherapy agents.
Biomedical waste does not include waste that is:
- from animal husbandry;
- household in origin;
- controlled in accordance with the Health of Animals Act
- generated in the food production, general building maintenance, and office administration activities of those facilities to which this applies.
Biomedical Waste Generators:
The Workplace Hazardous Materials Information System (WHMIS), makes it mandatory that all hazardous substances, including microorganisms, e.g., those used in research or other pursuits, be labelled in a specified manner and that a Material Safety Data Sheet (MSDS) be available to accompany each hazardous substance. Currently, the requirements of WHMIS do not apply to waste materials. All such Acts require the employer to provide information, instruction, and supervision to workers to protect their health and safety, and take every reasonable precaution in circumstances to protect the worker. Employers must provide all training necessary to work with hazardous substances and must keep a written record of their employee education program.
Policies and Procedures:
Workers handling and disposing of biomedical waste are at potential risk of exposure to infection from sharps-related accidents or when containers of waste burst open and leak, or spills of certain waste materials occur. Facilities and organizations responsible for waste handling and disposal should take reasonable steps to reduce the risk of exposure to infection by establishing written policies and procedures based upon the most currently accepted clinical and occupational health and safety information. Workers handling and disposing of biomedical waste should participate in the preparation of these policies and procedures. Policies and procedures should be reviewed and updated regularly, with compliance to their requirements verified as necessary.
Employee training programs must emphasize the following:
- personal hygiene, especially washing hands;
- the facility’s procedures for the reduction, segregation, collection, packaging, color coding, labeling, storage, and in-house movement of waste;
- methods for preventing the transmission of infections related to waste-handling procedures;
- the hazards of those materials to which workers may be exposed; and
- the actions to be taken and which supervisory staff should be notified in the event of an accident.
Employee training programs should be continually assessed and reinforced, and their content periodically reviewed and updated as necessary. Consideration should be given to adapting the training programs to suit personnel who may not be fluent in the official language of predominant use or who may not be fully literate.
Color-coding and Labelling:
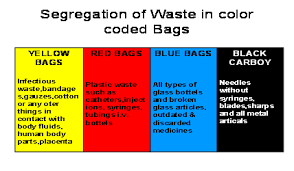
Containers for biomedical waste must be colour-coded as shown in Table 2 and labelled with the bio hazard symbol .This must be implemented as part of each health care facility’s biomedical waste management program.
Waste Type Color-coding
Human Anatomical ———RED
Animal Waste—————-ORANGE
Microbiology Laboratory Waste—–YELLOW
Human Blood and Body Fluid Waste (if applicable)——-YELLOW
Waste Sharps——YELLOW
Containers for biomedical waste must be color-coded by:
dyeing the entire container in the appropriate color; encircling the outer surface of the container with a band of color not less than 50 mm wide; or other methods to ensure staff recognition. If a sharps container is mounted in a cabinet or some other type of holder, only the actual sharps container must be color coded and labelled with the bio hazard and cytotoxic symbols, as appropriate. The outer cabinet or holder must, however, be labelled as containing sharps, using the words “CAUTION: WASTE SHARPS”, or an equivalent.
Bio hazardous Waste Labels, Bags, and Containers
Medical/bio hazardous waste generated must be disposed of in bio hazardous waste bags, as discussed in detail in the following sections. Bio hazardous waste bags must be placed in labeled bio hazardous waste containers.
Bio hazardous Waste Labels:
Bio hazardous waste labels with either the words “Bio hazardous Waste,” or with a bio hazard symbol and the word “Bio hazard” (see Figure -1) must be placed on bio hazardous waste containers.
Figure-1. A bio hazardous waste label with the bio hazard symbol.
Bio hazardous Waste Bags:
Bio hazardous waste bags must be either RED or clear (orange bags are not allowed) and labeled with either the words “Bio hazardous Waste,” or with a bio hazard symbol and the word “Bio hazard.” These bags must be disposable and impervious to moisture, and have strength sufficient to preclude ripping, tearing, or bursting under normal conditions of usage and handling.
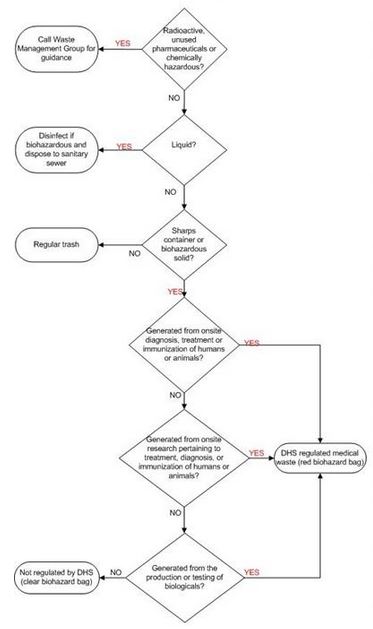
Red bio hazard bags must be used for regulated Medical Waste, which is regulated by the Department of Public Health (DPH). Regulated Medical Waste is generated or produced as a result of any of the following:
- Diagnosis, treatment, or immunization of human beings or animals
- Research pertaining to treatment, diagnosis, or immunization of human beings or animals or
- The production of biological
Red bio hazard bags are used to line all medical/bio hazard containers in laboratories where any regulated medical waste is produced, and red bio hazard bags are used to line all pickup containers provided by the disposal contractor. Use of red bio hazard bags is identified in the Bio safety Work Authorization. If a room uses red bio hazard bags, it will be assumed the waste is regulated and must conform to the red-bag requirements listed after the bio hazardous/medical waste disposal flowchart shown in Figure 2.
Clear bio hazard bags are used for bio hazardous waste that is not regulated by the Department of Public Health (DPH) but may be regulated by other bio safety standards. A laboratory can use clear bio hazard bags only when the Bio safety Work Authorization has identified a laboratory’s bio hazardous waste as non-regulated waste.
Red bio hazard bag Clear bio hazard bag.
The color of a bio hazardous bag is used to differentiate between DPH-regulated bio hazardous waste (red), and non-DPH-regulated bio hazardous waste that may be regulated by other standards (clear). The color of the bag does not indicate the level of biological risk or final treatment.
Biohazardous/Medical waste disposal flow chart
Figure – 2
Segregation:![]()
Whether the method of disposal is on-site or off-site, biomedical waste must be segregated from the general waste stream. If biomedical waste is mixed with general refuse, the total waste stream would require special treatment and handling. Waste segregation relies on the waste being segregated at its point of generation and placed into appropriate waste containers. Segregation permits facilities to effectively divert those materials that are recyclable, require special handling or disposal.
Biomedical waste must be segregated at the point of generation into the following waste categories: human anatomical waste; animal waste; microbiology laboratory waste; human blood and body fluid waste; and waste sharps.
Packaging:
Waste must be safely contained during handling and to the point of its disposal. The packaging must remain intact throughout handling, storage, transportation, and treatment. When selecting packaging, the following factors should be considered: the type of waste being contained; appropriate colour-coding and labelling (see color coding section ); special transportation requirements; the method of disposal; transport requirements; and requirements of the disposal facility.
To simplify their selection and use, waste containers should be classified as reusable or single-use/disposable.
Reusable Containers:
Reusable waste containers must be made of metal or rigid plastic and able to withstand exposure to common cleaning agents. They must be colour-coded according to the type of waste for which they are intended ; and labelled with the biohazard symbol (Figure 1).
Reusable waste containers should be inspected for holes or leaks each time they are emptied and their colour coding and labelling renewed if necessary. Holes or leaks must be repaired or the waste container replaced. Reusable waste containers must be cleaned regularly to prevent odours and as soon as possible if waste materials leak or spill within the containers.
Single-use Containers:
Single-use waste containers should be classified as one of the following types: sharps container; waste-holding plastic bag; or cardboard container.
Sharps Containers – The critical characteristic of any sharps container is that it be sturdy enough to resist puncture under conditions of use and to the point of disposal. Until a method is devised to determine this objectively, sharps containers should be tested and evaluated under actual conditions of use.
Storage of Biomedical Waste:
After biomedical waste has been collected and moved from its point of generation, it may be held in storage areas to await disposal. These storage areas must be totally enclosed, and separate from supply rooms or food preparation areas. They must be lockable and access must be restricted to authorized personnel. Storage areas must be identified as containing biomedical waste, with the biohazard symbol clearly displayed. It is unacceptable for materials other than waste to be placed in the same storage area as biomedical waste.
Floors, walls, and ceilings of storage areas must be thoroughly cleaned in accordance with the facility’s established procedures. These procedures should be prepared in consultation with the facility’s infection control committee, biosafety officer, or other appointed person(s).
Anatomical wastes must be stored at 4oC or lower. All biomedical waste must be refrigerated at 4oC or lower if stored for more than four days. Health care facilities should determine the maximum storage time of refrigerated or frozen biomedical waste based upon its storage capacity and rate of waste generation.
Facilities refrigerating or freezing stored waste should use a lockable, closed cold storage facility or a lockable, domestic type freezer unit. Either type must be used only for storing biomedical waste, visibly display the biohazard symbol, and be identified as containing biomedical waste.
Contingency plans must be prepared for storing refrigerated biomedical waste if excess waste is produced, or if either refrigeration or disposal facilities or equipment become in operative.
The compaction or shredding of untreated biomedical waste is not permitted unless the compactor or shredder is an integral part of the incinerator and completely sealed.
In-house Movement of Wastes:![]()
The handling and transport of waste containers should be minimized to reduce the likelihood of exposure to the waste.
Careful selection of waste containers greatly reduces the likelihood of breakage and leakage during use. In anticipation of such accidents occurring, however, a materialhandling system should be devised to minimize the possibility of inadvertent exposure by limiting the amount of handling. Specific routes must be planned through the facility to minimize the passage of loaded carts through patient care and other clean areas.
To minimize the possibility of waste handlers incurring injuries while handling filled waste containers, the facility’s health and safety committee, biomedical waste management committee or other appointed person(s) should establish size and weight criteria for the waste loads.
Carts used for moving biomedical waste through the health care facility should be designed to prevent spills, and made of materials able to withstand exposure to common cleaning agents.
These carts must be thoroughly cleaned before any maintenance work is performed on them. They should be cleaned regularly to prevent odours and as soon as possible if waste materials leak or spill in the carts.
The facility’s infection control committee, biosafety officer, or other appointed person(s) should be consulted about the frequency of cleaning and the type of cleaning agent tobe used.
Laboratory Biohazardous Waste Containers:
Biohazardous waste containers (Figure-3) must be rigid and leakproof, and must have a tight-fitting lid. Containers with foot pedals to open and close the lid are preferred. The containers may be any color, but they must be labeled with either the words “Biohazardous Waste,” or with a biohazard symbol and the word “Biohazard.” The labels must be placed on both the lid and the sides of the container. The labels must be visible from all sides of the container. In addition, biological materials of human origin that are covered by the OSHA Bloodborne Pathogen Standard must be placed in red containers, or in containers that have fluorescent orange or orange-red biohazard labels.

Figure-3- A biohazardous waste container.
Biohazardous waste containers must be lined with biohazardous waste bags before adding the waste. The labels on the container must be visible once a biohazardous waste bag is added. In general, lids should be used to prevent the spread of potentially infectious agents or material. The lid should be kept closed on the container whenever waste is not being actively added to the bag. At a minimum, the lid must be on the container during breaks, lunch, and at the end of each workday. Small countertop containers lined with clear bags (used for nonregulated biohazardous waste) can be used and kept uncovered. Larger containers lined with clear biohazard bags should be covered as a best-management practice.
Medical/Biohazardous Waste Pickup Containers:
The waste is collected from specified containers called pickup containers (Figure-4). The pickup containers are supplied by the medical/biohazardous waste subcontractor and are usually gray in color, except for red pathology containers, which are discussed later. They are prelabeled with biohazard symbols and the word “Biohazard.” Medical/biohazardous waste collected in laboratory waste containers (red-bagged or clear-bagged) must be transferred to these pickup containers for pickup. Laboratory waste in red bags must be transferred weekly. Laboratory waste in clear bags need only be transferred when the bag is full, when there is a noxious odor, or when continued accumulation may present a biohazard to personnel.
Figure-4 Medical/ biohazardous waste pickup containers
Solid Medical/Biohazardous Waste Disposal:
This section describes the procedure that must be followed when transporting waste from laboratory medical/biohazardous waste containers to medical waste pickup containers. This includes moving medical/biohazardous waste more than a few feet within a room.
Wear and use personal protective equipment (PPE) appropriately when handling medical/biohazardous waste (Figure -5). Wear PPE (e.g., lab coat, gloves, safety glasses) to prevent potential contact with and exposure to infectious material. In addition, prevent the spread of infectious material by:
a) changing gloves that have been used or may be contaminated,
b) not touching doorknobs or other “clean” surfaces with gloved hands, and
c) washing hands after removing gloves.
Seal the biohazard bag closed (tape, rubber band, etc.). Carry the biohazard bag to the nearest medical waste pickup container (Figure -6). The bio hazard bag must be secondarily contained during transport in a labeled biohazard container with a lid.
This is a necessary precaution, should the bag leak. Remove the biohazard bag and deposit it into the pickup container. The pickup container must be lined with a red biohazard bag. Close the lid on the pickup container after adding the waste.
Note: Do not overfill the gray pickup containers. The lid must be able to fully close. Start a new one if necessary. Wash your hands after removing your gloves.
Figure -5 Figure -6
Liquid Medical/Biohazardous Waste Disposal:
Many liquid medical/biohazardous wastes (e.g., cell cultures or blood) that are not chemical hazardous waste or radioactive waste can be sufficiently decontaminated and then poured down a sanitary sewer drain (e.g., laboratory sink drain).
Remember the waste must not be defined as chemically hazardous or radioactive before drain disposal or cause a violation of the wastewater permit discharge limits. Contact the Environmental Services Group for assistance with this type of waste.
Decontaminate and dispose of liquid medical/biohazardous waste as follows:
1. Add household bleach to the liquid to be decontaminated until a 10% (1:10) concentration of the household bleach is achieved.
2. Allow the bleach to remain in contact with the liquid waste material for approximately 20 minutes.
3. Dispose of the decontaminated liquid medical/biohazardous waste down the sanitary sewer drain.

Figure -7 To disinfect your liquid waste, create a 10% concentration of household bleach
in the liquid waste.
Disposal According to Type of Waste:![]()
Human Anatomical Waste
Human anatomical waste, consisting of human tissues, organs, and body parts, but excluding teeth, hair, and nails, must be incinerated in a biomedical waste incinerator or destroyed in a crematorium incinerator.
Animal Waste
Most animal waste, with the exception of teeth,hair, nails, hooves,and feathers, should be incinerated in a biomedical waste incinerator. This includes all animal tissues, organs, body parts, carcasses, bedding, fluid blood and blood products, items saturated or dripping with blood, body fluids contaminated with blood,and body fluids removed for diagnosis or removed during surgery, treatment or autopsy, unless a trained person certifies that the waste does not contain the viruses and agents listed.
Microbiology Laboratory Waste
Microbiology laboratory waste consisting of laboratory cultures, stocks or specimens of microorganisms; live or attenuated vaccines; human or animal cell cultures used in research; and laboratory material that has come into contact with the above, must be incinerated, autoclaved, or chemically disinfected.
Human Blood and Body Fluid Waste![]()
Except for those wastes associated with the exotic communicable diseases, fluid human blood and blood products, body fluids contaminated with blood, and body fluids removed for diagnosis or removed during surgery, treatment or autopsy, but excluding urine or feces, may be poured down the sanitary sewer after steam autoclave or chemical decontamination. The approval of the community works department and local Health Board is required. If approved, the treated waste should be carefully poured down a drain connected to the sanitary sewer.
When handling these fluids, care must be taken to eliminate spills and the formation of aerosols. At no time should these fluids be disposed of to the storm sewer.
Waste Sharps:
Clinical and laboratory materials consisting of needles, syringes, blades, or laboratory glass capable of causing punctures or cuts (referred to as waste sharps), must be incinerated. They may be autoclaved or chemically disinfected prior to incineration. When autoclaved, sharps containers must remain functionally intact at high autoclaving temperatures.
Mercury in Dental Amalgams:![]()
Dentist offices routinely generate waste or excess dental amalgam from dental repairs. Disposal of this mercury containing amalgam into the sanitary sewer or municipal refuse collection system is an unacceptable practice. Environmental conditions at sewage lagoons or landfill can react with the amalgam and release the mercury into the environment.
Mouth wash and aspiration equipment should be equipped with ISO certified amalgam traps capable of a 95% capture rate. Maintenance of the mercury traps is also important to ensure collected mercury does not overload the collection system defeating the cost and installation of the equipment. Efforts should also be made to replace dental amalgam with non-mercury composite materials, where possible, to reduce amalgam waste.
All amalgam collected from dental offices must be managed as a hazardous waste. Disposal of articles contaminated with or containing mercury by any means other than shipment to an approved chemical management facility, mercury recycler or the product manufacturer is in contravention of the EPA.