What are chemical substances:
A chemical substance is a material with a specific chemical composition. Water is an example of a chemical substance – it always has the same number of hydrogen and oxygen (e.g. H2O). Some everyday chemicals include cleaning materials, cosmetics, plastics, paint, dyestuffs, sugar, solvents, etc. A chemical substance can exist as a solid, liquid or gas and still be the same substance. For example, water and steam are different forms of the same substance.
Form of chemicals:
Chemicals are present in every workplace. Even in the cleanest, most modern office, employees may be routinely exposed to inks, toners and adhesives not to mention a wide range of chemicals used in cleaning and maintenance.
Chemicals can exist in many forms:
- Dust, fumes, fibres, powders.
Any chemical, in either gas, liquid or solid form, that has the potential to cause harm is referred to asa hazardous or dangerous chemical. Such chemicals include those:
- Brought directly into the workplace and handled, stored and used for processing e.g. solvents, cleaning agents, glues, resins, paints.
- Generated by a process or work activity e.g. fumes from welding/ soldering, dust from machining of wood, flour dust, solvents.
- Generated as waste or residue e.g. fumes from soldering iron, carbon monoxide from engine or motor exhausts.
Chemicals be hazardous to health:
Chemicals can cause many different types of harm, ranging from mild skin irritation to cancer. The effects of hazardous chemicals may be seen:
- Chronic :Immediately after contact (e.g. chemical burn) or many years after the exposure (e.g. lung cancer following exposure to asbestos).
- Acute :Following a single short exposure (e.g. infrequent use of a chemical) or longer-term exposures (e.g. daily use of a chemical in the workplace).
- Algergy :The effect is exerted through the immune system (multiple initial doses result in sensitization with the accumulation of antibodies; subsequent low-level exposure triggers a response; pronounced individual susceptibility) e.g. respiratory and skin sensitizes (chrome, nickel, platinum salts).
Therefore, it is important to minimise exposure to chemicals at all times. In order for a chemical to be hazardous to a person’s health, it must either be in contact with or enter the body.
Risk from chemical exposure:

If the chemicals that are used in your workplace are not properly controlled, then it is possible you are being exposed to them.Whether this exposure causes you any harm depends on a combination of factors:
- how hazardous the chemical is;
- how long you are exposed to the chemical (e.g. 5 minutes, 3 hours);
- how often you are exposed (e.g. twice a week/month)
Here are some examples of how chemicals can affect the body.
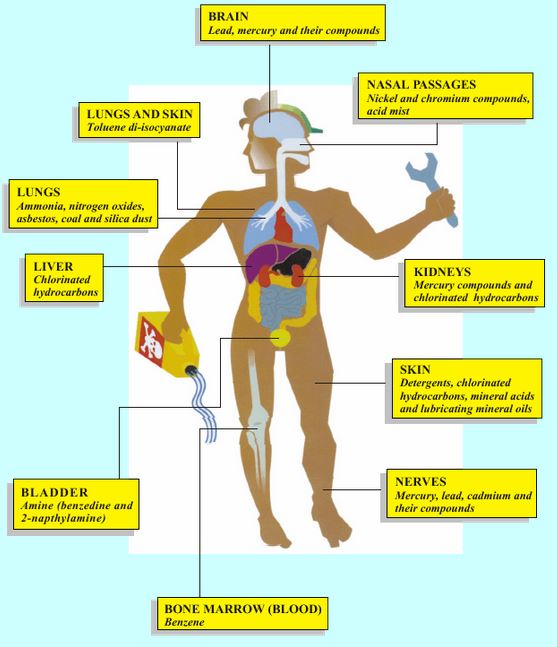
Effects on brain and nervous system For example, exposure to pesticides, mercury, lead, solvents, carbon monoxide gas.
Eye, nose and throat irritation (dryness, soreness or pain) For example, exposure to acid mists and vapours, welding fumes or diesel exhaust.
Effects on the lung Lung damage For example asbestos (lung cancer), welding fume (chronic obstructive pulmonary disease).
Irritant induced asthma For example acids (“burn effect” on airways).
Allergic asthma For example flour dust,isocyanate (in 2-pack paints), wood dust 
Liver damage For example, exposure to vinyl chloride.
Bladder damage For example, exposure to some azo dyes (bladder cancer).
Effects on skin
Allergic contact dermatitis For example nickel, latex, chromate (found in some cements).
Irritant contact dermatitis For example solvents, detergents, oils, lubricants.
Effects on blood and bone marrow For example, exposure to benzene in petrol fumes (anemia and leukaemia).
chemicals effect the body:
You have seen how chemicals effect the body. There are four ways chemicals can enter the body:
Inhalation:
Breathing in contaminated air is the most common way that workplace chemicals enterthe body.
Contact with the skin or eyes:
Some chemicals can damage the skin or eyes (e.g. irritation) or pass through the skin into the body.
Ingestion:
Workplace chemicals may be swallowed accidentally if food or hands are contaminated.
Injection:
Injection can occur when a sharp object (e.g. needle) punctures the skin and injects a chemical directly into the bloodstream.